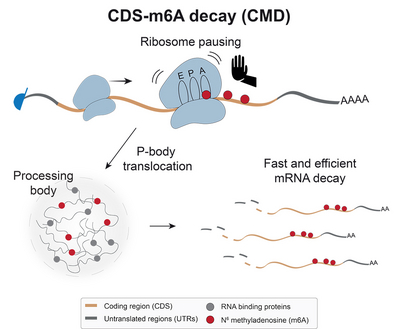
How adenosine methylation in the mRNA coding region triggers gene silencing
The team of Dr Julian König, a Group Leader at the Institute of Molecular Biology (IMB) in Mainz, Germany, has discovered how cells silence their genes by adding an RNA modification called adenosine methylation (m6A) to the coding region of their mRNAs. Julian’s team found that the presence of m6A in this region causes ribosomes to pause, slowing down translation, and triggers a previously unknown, highly efficient mRNA degradation pathway. This silencing mechanism may be especially important for controlling the expression of developmental genes and retrotransposons. Their findings were published in the journal Molecular Cell.
For cells to function properly, each gene must be expressed at the right time and just the right amount. This is achieved by fine-tuning the expression of RNAs and proteins through numerous mechanisms. One such mechanism is RNA modifications – specifically the methylation of adenine bases (m6A) in the RNA. m6A reduces the stability of messenger RNAs (mRNAs), accelerating their degradation so that there is less time for them to be translated, and thus less protein produced. However, until now scientists did not know whether the position of m6A on the mRNA had any significance.
Now Julian and his research team and their collaborators in the Zarnack lab at the University of Würzburg (previously Goethe University Frankfurt) have shown for the first time that cells can quickly silence genes by adding m6A specifically to the coding region of mRNAs, which triggers their rapid degradation via a previously unknown pathway.
The researchers first compared the expression and stability of mRNAs with m6A in different locations. To their surprise, mRNAs with m6A in the coding region were much less stable than other mRNAs with m6A in untranslated regions. The more m6A sites in the coding region, the less stable the mRNA became. "The prominent effect of m6A sites in the coding region was surprising since m6A sites had traditionally been regarded as equally functional along mRNAs", says first author Dr You Zhou from the Zarnack lab.
Since the coding region is particularly important for translating the mRNA into protein, Julian and his colleagues wondered if m6A somehow impacted translation. To investigate this, Julian and his team mapped out the locations of translating ribosomes on mRNAs containing m6A in the coding region and then looked at what happened when they removed m6A with a specific inhibitor. When m6A was present, they saw many ribosomes crowded around m6A sites, while removing m6A eliminated this crowding. This suggested that translating ribosomes may slow down around m6A sites. "The direct coupling of translation to mRNA decay offers an elegant means to tightly control the protein output of mRNAs", says Julian.
Further investigation revealed that many of these m6A-containing mRNAs were located in P-bodies – small compartments in the cell known to contain RNA degradation proteins and silenced mRNAs. "A link to P-bodies had been suggested in the past, although the mechanism had remained unclear", says co-first author Dr Peter Hoch-Kraft. Based on their observations, the researchers hypothesise that m6A may cause translating ribosomes to pause on the mRNA and that this may trigger its translocation to P-bodies for degradation. Julian and his colleagues named this new pathway “coding sequence (CDS)–m6A decay”.
As many of the mRNAs targeted by this pathway were from genes involved in development or from retrotransposons, the authors hypothesise that CDS–m6A decay may be especially important for controlling genes during development and protecting the genome against the activation of retrotransposons. "Given that m6A inhibitors have already been tested in clinical trials, I am excited about the potential to use our findings for targeted interventions in the future", says co-first author Miona Ćorović.
Julian and his team next aim to study which proteins are involved in CDS–m6A decay, how cells sense ribosome pausing at m6A sites on the mRNA, and how this may trigger its subsequent transport to P-bodies. Such findings will help scientists to better understand how cells regulate their genes, as well as what goes wrong in disease.
Further details
Further information can be found at https://doi.org/10.1016/j.molcel.2024.10.033
Julian König is a Group Leader at the Institute of Molecular Biology. Further information about research in the König lab can be found at www.imb.de/koenig.
About the Institute of Molecular Biology gGmbH
The Institute of Molecular Biology gGmbH (IMB) is a centre of excellence in the life sciences that was established in 2011 on the campus of Johannes Gutenberg University Mainz (JGU). Research at IMB focuses on the cutting-edge fields of epigenetics, genome stability, ageing and RNA biology. The institute is a prime example of successful collaboration between a private foundation and government: The Boehringer Ingelheim Foundation has committed 154 million euros to be disbursed from 2009 until 2027 to cover the operating costs of research at IMB. The State of Rhineland-Palatinate has provided approximately 50 million euros for the construction of a state-of-the-art building and is giving a further 52 million in core funding from 2020 until 2027. For more information about IMB, please visit: www.imb.de.
Boehringer Ingelheim Foundation
The Boehringer Ingelheim Foundation is an independent, non-profit organization that is committed to the promotion of the medical, biological, chemical, and pharmaceutical sciences. It was established in 1977 by Hubertus Liebrecht (1931–1991), a member of the shareholder family of the Boehringer Ingelheim company. Through its funding programmes Exploration Grants, Plus 3, and Rise up!, the Foundation supports excellent scientists during critical stages of their careers. It also endows the international Heinrich Wieland Prize, as well as awards for up-and-coming scientists in Germany. In addition, the Foundation funds institutional projects such as the Vienna-based AITHYRA institute combining AI and Biomedicine, the Institute of Molecular Biology (IMB) and the European Molecular Biology Laboratory (EMBL), both in Germany.
Press contact for further information
Dr Ralf Dahm, Director of Scientific Management
Institute of Molecular Biology gGmbH (IMB), Ackermannweg 4, 55128 Mainz, Germany
Phone: +49 (0) 6131 39 21455, Email: press(at)imb.de