Kubben Lab
Research Publications Group Members BiographyBiology of ageing & ageing-related diseases
Our lab is broadly interested in unravelling the molecular mechanisms that underlie human ageing and finding ways to limit their impact on disease, helping increase human health- and lifespan.
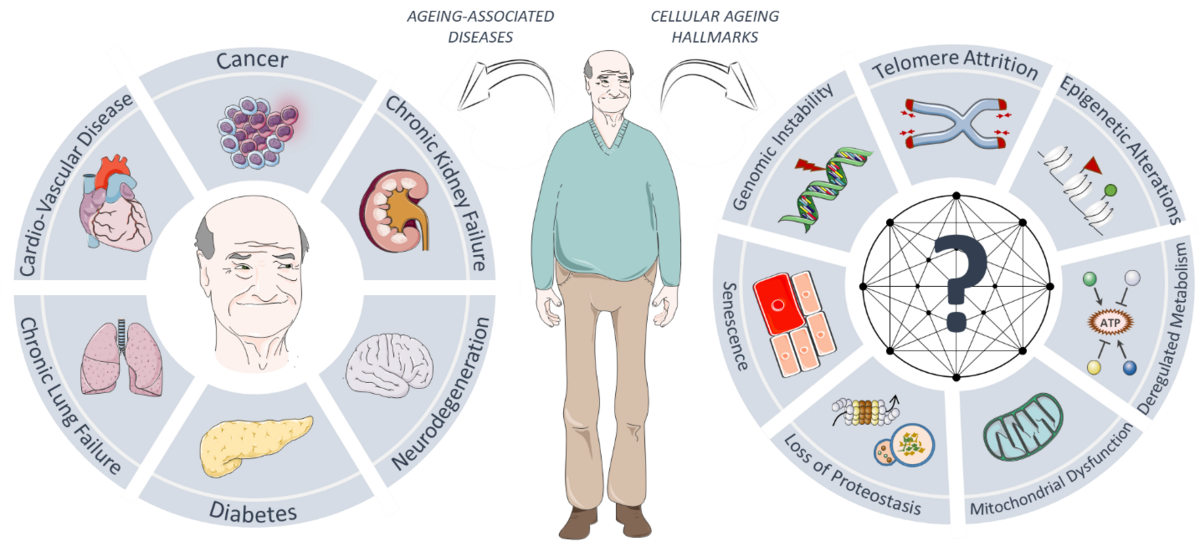
Ageing is a prime pathological component of most prevalent diseases, such as cancer, diabetes, cardiovascular, neurodegenerative and chronic kidney and lung diseases. At the cellular level, ageing is characterised by several hallmarks, including epigenetic alterations, genomic instability and loss of protein homeostasis (i.e. proteostasis), that lead to an organism-wide functional decline. Recent studies have provided proof-of-principle that anti-ageing interventions hold great potential in limiting the devastating impact of cellular ageing on human health- and lifespan. Unfortunately, our current knowledge of the molecular pathways that control cellular ageing, as well as which modulations can help delay the onset of ageing and ageing-related diseases, is severely limited. This knowledge gap is brought about in part by a major long-term challenge in the field of ageing: the lack of robust model systems of human ageing that allow us to identify and distinguish so-called drivers of ageing from bystander effects in a high-throughput and unbiased manner. Recent technological advances in high-throughput microscopy and genome-wide screening platforms, as well as the identification of the genetic cause underlying the rare premature ageing disease Hutchinson-Gilford Progeria Syndrome (HGPS), have now provided us with exciting new opportunities to further unravel the pathomechanistic basis of human ageing.
Hutchinson-Gilford Progeria Syndrome (HGPS)
HGPS is an extremely rare premature ageing disease in humans that is invariably fatal by the age of about 15. While children with HGPS are born relatively healthy, they age approximately 6 times faster than the average healthy child. The most common causes of death for children with HGPS include atherosclerosis-resembling progressive cardiovascular disease, myocardial infarction, and stroke. HGPS is predominantly caused by a heterozygous silent mutation (G608G) in the LMNA gene, which encodes lamin A – a nuclear lamina protein and a key organiser of mammalian nuclear structure and function. The G608G mutation activates a cryptic splice-donor site that results in the production of an aberrant lamin A isoform called progerin. It remains largely unknown how the accumulation of progerin exerts its dominant negative ageing effects.
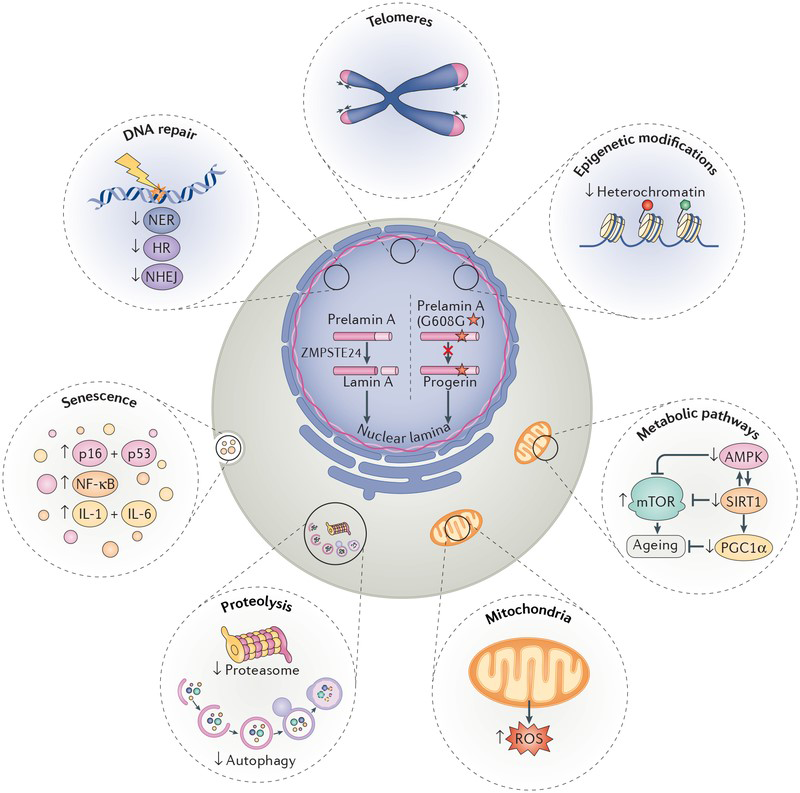
The synthesis of an aberrantly spliced isoform of lamin A called progerin is sufficient to bring about most classically-defined hallmarks of cellular ageing.
HGPS patients and normal, physiologically aged individuals develop remarkably similar ageing-related pathologies. Cells from HGPS patients display many classical hallmarks of ageing, including genomic instability, loss of heterochromatin and proteostasis, impaired mitochondria, and activation of the senescence pathway. This resemblance may be partially explained by the fact that normal, physiologically aged individuals also synthesise small amounts of progerin, due to sporadic use of the same cryptic splice site that is hyper-activated by the G608G lamin A mutation in HGPS patients. HGPS is a paradigmatic model to study ageing because (1) the genetic defects in HGPS patients are well defined, (2) there are direct links between progerin and numerous hallmarks of cellular ageing, (3) the ageing-related pathologies in HGPS manifest across multiple tissues, and (4) progerin is also expressed during normal physiological ageing.
Our Research
Within our lab, we have genetically engineered a cell system in which we can chemically induce and titrate progerin expression, enabling us to control the onset, duration and severity of the cellular cascades that drive the formation of most well-known ageing hallmarks. Ageing defects that normally take decades to form during physiological ageing can now be induced and studied in less than 4 days. This system is a breakthrough in ageing research, as it captures early events in the ageing process, is genetically tractable, and enables unbiased identification of molecular processes that trigger and propel cellular ageing while allowing them to be distinguished from bystander effects. Coupling this cellular system to high-throughput, high-content imaging approaches and genome-wide screening technologies (RNAi, CRISPR) has provided proof-of-concept that we can identify novel anti-ageing pathways that hold therapeutic potential. Some of our main ongoing lines of investigation focus on:
- Characterising the molecular mechanisms by which progerin disrupts cellular proteostasis
- Genome-wide screening of molecular mechanisms that drive cellular ageing
- In vivo identification of therapeutic anti-ageing interventions for HGPS and other ageing-associated diseases
To investigate these topics, we use complementary approaches, including state-of-the-art high-throughput microscopy, high-content image analysis, robotics-automated immunofluorescent staining, genome engineering (CRISP/Cas9), FACS, biochemistry assays, affinity purification, transcriptomics, and proteomic techniques.
Recruiting
Our lab is growing into a multi-cultural, dynamic group of researchers who are intellectually, technically, and socially driven to make an impact in translational ageing research. We strive to provide consistent mentoring and continuous support for our team members’ career development. If you want to join our team, we welcome your application by sending us your CV and a brief motivation letter by email.